April 13, 2014
I have prevously written about the serious problems with the way that the FDA has done cost-benefit analysis of the effects of tobacco control policies, most notably warning labels on cigarette packages. Among other things, the FDA, led by economist Clark Nardinelli, assumes that steps that will reduce smoking have the effect of depriving smokers (and potential smokers) of the "pleasure" of smoking, quantified using the economic idea of "consumer surplus." Counting this lost pleasure as a cost led the FDA to substantially discount any health benefits of stopping (or not starting smoking), which made the regulation harder to defend in court..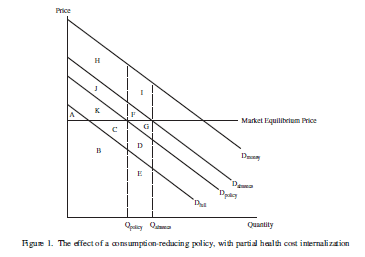
April 13, 2014
Greg Connolly and Hillel Alpert recently published an important paper in Tobacco Control, "Has the tobacco industry evaded the FDA's ban on ‘Light’ cigarette descriptors?," that presents empirical evidence that the ciagrette companies have effectively nullified the ban on selling cigarettes with the misleading descriptors "light" and "mild" that are in both the Family Smoking Prevention and Tobacco Control Act and Judge Gladys Kessler's RICO decision.
Here is the abstract from the paper:
Background Under the Family Smoking Prevention and Tobacco Control Act (FSPTCA), the Food and Drug Administration (FDA) banned the use of “Lights” descriptors or similar terms on tobacco products that convey messages of reduced risk. Manufacturers eliminated terms explicitly stated and substituted colour name descriptors corresponding to the banned terms. This paper examines whether the tobacco industry complied with or circumvented the law and potential FDA regulatory actions.
Methods Philip Morris retailer manuals, manufacturers' annual reports filed with the Massachusetts Department of Public Health, a national public opinion survey, and market-wide cigarette sales data were examined.
April 5, 2014
On April 4, 2014, the FDA announced that it will review all "regular" substantial equivalence submissions by tobacco companies immediately, responding to industry complaints that the process is taking a long time. (A substantial equivalence application is one in which the company claims that a new product they want to market is "substantially equivalent" to one already on the market so there is no need for a full new product review. As I noted before substantial equivalence is a huge loophole in the law .)
It is understandable why the industry wants regular SE applications processed quickly, since “Regular SE applications” are for products that did not enter the market between Feb. 16, 2007 and March 21, 2011, SE reports were filed after March 22, 2011, and which can not be marketed until FDA determines that they are substantially equivalent.
April 4, 2014
On March 28, 2014, Lancet published "Effect of smoke-free legislation on perinatal and child health: a systematic review and meta-analysis," which found that "Smoke-free legislation was associated with reductions in preterm birth (four studies, 1 366 862 individuals; −10·4% [95% CI −18·8 to −2·0]; p=0·016) and hospital attendances for asthma (three studies, 225 753 events: −10·1% [95% CI −15·2 to −5·0]; p=0·0001). No significant effect on low birthweight was identified (six studies, >1·9 million individuals: −1·7% [95% CI −5·1 to 1·6]; p=0·31)."
In an accompanying commentary, "Smoke-free policies: Clearing the air with money to spare," Sara Kalkohoran and I observed that this paper added to the already-strong evidence that smokefree laws were followed by immediate drops in hospital admissions for heart attacks, strokes, and other cardiopulmonary conditions. All this evidence means that smokefree laws have large and rapid benefits in terms of improved health and, as a result, contribute toi medical care cost containment.
April 3, 2014
The CDC Morbidity and Mortality Monthy Report just reported numbers showing that e-cigarettes are accounting for a big increase in calls to poison control centers.
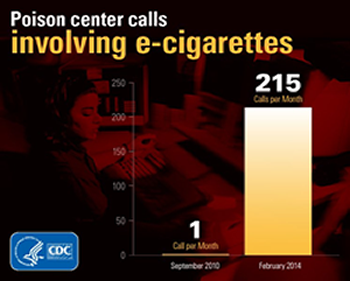 Here are key statements from the report:
Here are key statements from the report:
E-cigarettes accounted for an increasing proportion of combined monthly e-cigarette and cigarette exposure calls, increasing from 0.3% in September 2010 to 41.7% in February 2014... Cigarette exposures were primarily among persons aged 0–5 years (94.9%), whereas e-cigarette exposures were mostly among persons aged 0–5 years (51.1%) and >20 years (42.0%). E-cigarette exposures were more likely to be reported as inhalations (16.8% versus 2.0%), eye exposures (8.5% versus 0.1%), and skin exposures (5.9% versus 0.1%), and less likely to be reported as ingestions (68.9% versus 97.8%) compared with cigarette exposures (pMMWR April 4, 2014 / 63(13);292-293
The full MMWR report is available here.
