April 16, 2018
Stanton A. Glantz, PhD
FDA’s Nicotine Steering Committee should develop policies, regulations, and procedures that promote cessation and increase the use of proven therapies
My UCSF colleagues and I submitted this public comment to the FDA Nicotine Steering Committee. The tracking number is 1k2-92ml-1zcp . A PDF of the comment (which includes the footnote referencing which is lost in this blog post) is available here and and the two attachments are here and here.
FDA’s Nicotine Steering Committee should develop policies, regulations, and procedures that promote cessation and increase the use of proven therapies
Docket No. FDA–2018–N–0128
Janice Tsoh PhD, Dorie Apollonio, PhD, Sharon Hall, PhD, Maya Vijayaraghavan MD, Danielle Ramo PhD, Pamela Ling MD, Lauren L. Lempert JD, MPH, Stanton A. Glantz PhD
Tobacco Center of Regulatory Science
University of California, San Francisco
FDA’s Nicotine Steering Committee (Committee) was created to address the regulation of nicotine products with the goal of reducing tobacco use. The primary focus of the Committee is on the use of therapeutic nicotine for combustible tobacco product cessation. Composed of leaders from the Center for Drug Evaluation and Research (CDER) and the Center for Tobacco Products (CTP), the Committee seeks to align FDA’s centers across the Agency and to develop unified FDA positions on this and other cross-cutting issues that span recreational as well as therapeutic uses. We applaud FDA’s efforts to develop a comprehensive strategy to address these critically important issues, and offer the following recommendations for consideration by the Committee.
- FDA should facilitate investigational new drug applications for commercially available electronic nicotine delivery systems
Among many issues that will be addressed by the Committee, electronic nicotine delivery systems (ENDS) are being promoted and used for smoking cessation. However, as we discussed in another comment submitted to this docket, only limited data are available on the efficacy of ENDS when used specifically for smoking cessation, and they do not address the potential value of ENDS as a smoking cessation intervention as part of a clinically supervised quit attempt. Moreover, because of problems with the investigational new drug (IND) application process that restrict researchers’ ability to conduct clinical trials on commercially available ENDS, researchers have not been able to advise FDA on the potential benefits vs harms of ENDS for cessation purposes. FDA should make it possible for researchers to conduct clinical trials of commercially available electronic nicotine delivery devices for smoking cessation without requiring the same level of preclinical testing appropriately required for an investigational new drug.
- FDA’s approach to evaluating nicotine replacement therapies (NRT) should be complete cessation, not “harm reduction”
The goal of FDA’s approach to evaluating nicotine replacement therapies (NRT) should be complete cessation, not “harm reduction.” As we discussed at length in a comment previously submitted to FDA’s docket on FDA’s approach to evaluating NRT, the likely result of adopting the tobacco industry’s harm reduction frame would be continued smoking and increases in overall harm. No level of tobacco use by any method of consumption is safe, and reduction or shifting to another tobacco product should not be the goal or an acceptable outcome of any treatment regimen.
To date there is little scientific evidence supporting the harm reduction theory, and both short- and long-term rigorous studies are required to demonstrate potential benefits of harm reduction before the FDA advocates this as a public health strategy. Rather, complete tobacco cessation and complete abstinence from any alternative tobacco products should continue to be the desired endpoint.
Importantly, scientific evidence shows that NRT used without counselling is ineffective, or even harmful, in terms of promoting cessation. Combined use of NRT, especially with e-cigarettes and other so-called “innovative technologies,” is likely to be associated with dual use with other tobacco products in the market and used today and is more likely to facilitate nicotine addiction, rather than reduce addiction.,, Therefore, to ensure that it is effective, FDA should implement labeling requirements, public education, and regulations to ensure that use of NRT must be accompanied by effective counseling and cessation support, and should discourage dual use with other tobacco products.
FDA’s approach to NRT should be based on the following evidence-based points:
(1) there is no safe level of tobacco use;
(2) smoking or other tobacco product reduction should not be the goal or an acceptable outcome of any NRT treatment regimen;
(3) complete cigarette cessation and complete abstinence from any alternative tobacco products should be the desired endpoint;
(4) NRT used without counselling is ineffective, or even harmful, in terms of promoting cessation; and
(5) NRT labeling should include clear directions and warnings to direct users to use NRT with smoking cessation support and to use NRT with the end-goal of complete abstinence of any tobacco products.
3. FDA should employ evidence-based strategies to improve the efficacy of existing NRT treatment regimens to help people completely quit using tobacco products
When developing its comprehensive strategy to use NRT to help tobacco users quit completely, FDA should adopt and implement evidence-based strategies to help people not only initiate quit attempts, but also maintain long-term abstinence. We emphasize again that these strategies recognize that while NRT is a proven cessation intervention when combined with counseling, it is ineffective or even harmful when NRT is used without counseling., Significantly, the tobacco companies understand this reality, which is why they stopped fighting use of NRT long ago and have entered the NRT market themselves.
As we discussed at length in a public comment previously submitted to the Centers for Disease Control (CDC), FDA should use its authority over labeling, public and provider education, and other regulatory authority to implement the following proven strategies:
(1) Promote emerging technologies such as social media interventions
(2) Leverage clinician-extender or point-of-care technology tools
(3) Provide cost-effective cessation services such as interactive voice response systems
(4) Discourage provision of over-the-counter NRT and other cessation mediations unless they are tied to counseling
(5) Use market segmentation strategies used by tobacco companies to define communication audiences and reach high risk and disadvantaged populations more effectively
(6) Mount public education campaigns to counter unsupported claims that e-cigarettes as they exist and are used in the market today are an effective smoking cessation intervention and warn the public about the dangers of dual use of e-cigarettes and cigarettes
(7) Advocate for insurance coverage of cessation treatments and encourage healthcare systems to include smoking cessation as part of their mandatory risk management efforts
(8) Encourage tobacco-free policies in substance abuse treatment and promote NRT in mental health and other healthcare settings, in social service and community settings, and in prisons, military, and other institutional settings
4. FDA should not implement policies and programs to move cigarette smokers to ENDS for tobacco product cessation until FDA has facilitated pre-clinical and clinical research studies that determine the public health impacts of promoting ENDS for smoking cessation and what, if any, limitations are warranted on use of ENDS for tobacco product cessation to maximize benefit and minimize harm
FDA’s Nicotine Steering Committee staff manual states that the first responsibility of the Committee is to “develop a nicotine policy strategy that seeks to reduce tobacco use and move cigarette smokers to less harmful means of nicotine delivery through the use of therapeutic nicotine for tobacco product cessation.” While some studies suggest that this strategy has the potential to improve public health several studies published recently show that daily that e-cigarette use may have an overall negative effect on smoking cessation.
While there have been several studies published recently showing that daily users of high nicotine delivery e-cigarette systems quit more than people who do not use e-cigarettes, these studies also show depressed quitting or no effect of non-daily users of high delivery systems as well as “cig-alikes.” Because only a small minority of e-cigarette users (10-20%) are daily users of high delivery systems, the overall population health effect of e-cigarettes on smoking cessation remains negative, i.e., on average smokers who use e-cigarettes are less likely to quit than smokers who do not use e-cigarettes.
A 2016 meta-analysis that considered all the available evidence at the time (including some evidence that heavy users of high delivery systems quit more) found an overall negative effect of e-cigarette use on smoking cessation. Dr. Glantz has updated this meta-analysis as new studies are published, and the conclusion that, overall, e-cigarettes depress quitting has remained stable. (see figure below). The current meta-analysis found that overall, the odds of quitting are significantly reduced (OR 0.77, 95% CI 0.06-0.99) among smokers who use e-cigarettes compared to smokers who do not use e-cigarettes. This result is essentially the same as the 2016 meta-analysis (OR 0.72, 95% CI 0.57-0.91).
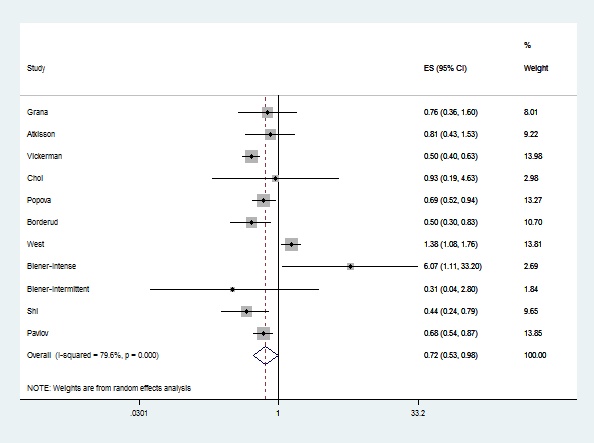
Moreover, there is a real question about whether randomized trials are even appropriate for assessing the effects of e-cigarettes on smoking cessation if they remain widely available as a recreational consumer product being sold by tobacco companies whose fundamental business goal is to maximize sales and profits. Randomized controlled trials, in which people are recruited using strict inclusion and exclusion criteria and randomized to receive treatments and then observed going forward, are widely accepted as the gold standard for assessing medicines which are administered and monitored by physicians or other health providers as part of a therapeutic effort. However, today e-cigarettes are not medicines. They are mass-marketed consumer products.
Population studies of use patterns and the effects of product use are more appropriate for assessing the effects of mass marketed consumer products because that reflects what is going on in the real world. Randomized clinical trials of e-cigarettes as part of a clinically supervised smoking cessation program would make sense (and current FDA rules make doing that impossible in practical terms), but then the results should only be applied to their use under medical supervision (and probably by prescription, given the high abuse potential).
This finding is important as the FDA’s Nicotine Steering Committee thinks about FDA’s comprehensive nicotine policy strategy, which seems to be premised on the idea that smokers should be pushed from combusted cigarettes to “less hazardous” other forms of nicotine delivery, particularly e-cigarettes. If e-cigarettes’ main effect for most people is to keep people smoking cigarettes, this policy could end up boomeranging and keeping people smoking cigarettes.
In addition, there is strong and consistent evidence that youth who initiate tobacco product use with e-cigarettes are much more likely to become cigarette smokers and that, among youth who are experimenting with cigarettes (smoked more than a puff but not 100 cigarettes), e-cigarette use is associated with progression to established smoking. There is also evidence that youth use of any non-cigarette tobacco product predicts subsequent cigarette smoking. At a population level, these effects on youth overwhelm any cessation benefits for adults associated with using e-cigarettes. The Nicotine Steering Committee must carefully consider these effects on youth when thinking about promoting these non-cigarette products as alternatives to conventional cigarettes. The issue, as required by the Family Smoking Prevention and Tobacco Control act must be on how these products are actually used rather than their theoretical value.
Because of the growing evidence of the high abuse potential and gateway effect of e-cigarettes, especially among youth and young adults, simply encouraging all people to use these systems is not appropriate for the protection of the public health and may be harmful to the public health as a whole, in violation of the Family Smoking Prevention and Tobacco Control Act’s section 907 mandates.
FDA must first make it possible for clinical trials, as is required for all FDA-approved drugs and devices, to be completed that demonstrate that high delivery nicotine systems or other ENDS are safe and effective cessation aids as actually used. (Even then, it would be difficult to ensure that these products would be used daily to achieve the desired outcome.) Because of their high abuse potential, the regulatory environment must be carefully crafted to ensure that these addictive products are used only for cessation, and not for recreational purposes. FDA should remain open to making approved ENDS available for smoking cessation as prescription drugs.
Based on these recent studies showing that most smokers who use e-cigarettes continue to smoke conventional cigarettes, FDA should not base its tobacco regulation and nicotine strategy on the premise that moving cigarette smokers to e-cigarettes or other non-combusted tobacco products will increase quitting unless and until:
- There are appropriate trials to ensure their safety and efficacy;
- The regulatory environment is carefully crafted to ensure that these products are used only for cessation; and
- The regulatory environment is crafted to effectively prevent youth initiation with any of these alternative products because of their gateway effect to conventional cigarettes.
FDA Staff Manual Guides, FDA Nicotine Steering Committee, SMG 2010.20. Available at: https://www.fda.gov/downloads/AboutFDA/ReportsManualsForms/StaffManualGuides/UCM594385.pdf
Benowitz N., Matthay M., St. Helen G., et al. FDA Should Facilitate Investigational New Drug Applications (IND) for Commercially Available Electronic Nicotine Delivery Devices, Comment submitted to Docket No. FDA-2018-N-0128, Nicotine Steering Committee; Establishment of a Public Docket; Request for Comments (April 12, 2018), tracking number 1k2-92jz-nsej. Available at: https://tobacco.ucsf.edu/sites/g/files/tkssra4661/f/wysiwyg/Develop%20IND%20for%20ENDS.12Apr2018.pdf
Apollonio D, Glantz SA, Hall S, et al. The FDA should not adopt the nicotine “harm reduction” paradigm because doing so is likely to increase the amount of smoking-caused disease and death. Comment submitted to Docket No. FDA-2017-N-6529, The Food and Drug Administration’s Approach to Evaluating Nicotine Replacement Therapies; Public Hearing; Request for Comments (Feb. 15, 2018). Available at: https://www.regulations.gov/document?D=FDA-2017-N-6529-0050 (Copy submitted with this comment)
Kotz D, Brown J, West R. Prospective cohort study of the effectiveness of smoking cessation treatments used in the “real world.” Mayo Clin Proc. 2014;89(10):1360–1367.
Kotz D, Brown J, West R. “Real-world” effectiveness of smoking cessation treatments: a population study. Addiction. 2014;109(3):491–499.
Leas EC, et al. Effectiveness of Pharmaceutical Smoking Cessation Aids in a Nationally Representative Cohort of American Smokers. JNCI: Journal of the National Cancer Institute, djx240, https://doi.org/10.1093/jnci/djx240. Published: 21 December 2017.
Kotz D, Brown J, West R. Prospective cohort study of the effectiveness of smoking cessation treatments used in the “real world.” Mayo Clin Proc. 2014;89(10):1360–1367. Kotz D, Brown J, West R. “Real-world” effectiveness of smoking cessation treatments: a population study. Addiction. 2014;109(3):491–499. Leas, EC, et al, Effectiveness of Pharmaceutical Smoking Cessation Aids in a Nationally Representative Cohort of American Smokers. JNCI: Journal of the National Cancer Institute, djx240, https://doi.org/10.1093/jnci/djx240. Published: 21 December 2017.
Apollonio D, Glantz SA. Tobacco Industry Research on Nicotine Replacement Therapy: "If Anyone Is Going to Take Away Our Business It Should Be Us". Am J Public Health. 2017 Oct;107(10):1636-1642. doi: 10.2105/AJPH.2017.303935. Epub 2017 Aug 17.
Apollonio D, Glantz SA. Tobacco Industry Research on Nicotine Replacement Therapy: "If Anyone Is Going to Take Away Our Business It Should Be Us". Am J Public Health. 2017 Oct;107(10):1636-1642. doi: 10.2105/AJPH.2017.303935. Epub 2017 Aug 17.
Tsoh J, Apollonio D, Gubner N et al. CDC should employ evidence-based strategies to help people quit using tobacco that support the initiation of quit attempts and maintaining long-term abstinence. Comment submitted to Docket No. CDC-2017-0103, Request for Information on Effective, Large-Scale, Sustainable Approaches to Help People Quit Using Tobacco by Employing Evidence-based Treatment Options (Jan. 2, 2018). Available at: https://www.regulations.gov/document?D=CDC-2017-0103-0019 (Copy submitted with this comment)
Apelberg BJ, Feirman SP, Salazar E et al. Potential Public Health Effects of Reducing Nicotine Levels in Cigarettes in the United States. N Engl J Med 2018 Mar 15. doi: 10.1056/NEJMsr1714617. [Epub ahead of print].
Berry KM, Reynolds LM, Collins JM, et al. E-cigarette initiation and associated changes in smoking cessation and reduction: the Population Assessment of Tobacco and Health Study, 2013–2015. Tobacco Control Published Online First: 24 March 2018. doi:10.1136/tobaccocontrol-2017-054108
Kalkhoran S, Glantz SA. E-cigarettes and smoking cessation in real-world and clinical settings: a systematic review and meta-analysis. The Lancet Respiratory medicine. 2016;4(2):116-128. doi:10.1016/S2213-2600(15)00521-4.
Benowitz, N., Matthay, M., St. Helen, G., et al., FDA Should Facilitate Investigational New Drug Applications (IND) for Commercially Available Electronic Nicotine Delivery Devices, Comment submitted to Docket No. FDA-2018-N-0128, Nicotine Steering Committee; Establishment of a Public Docket; Request for Comments (April 12, 2018), tracking number 1k2-92jz-nsej. Available at: https://tobacco.ucsf.edu/sites/g/files/tkssra4661/f/wysiwyg/Develop%20IND%20for%20ENDS.12Apr2018.pdf
Soneji S, Barrington-Trimis JL, Wills TA, Leventhal AM, Unger JB, Gibson LA, Yang J, Primack BA, Andrews JA, Miech RA, Spindle TR, Dick DM, Eissenberg T, Hornik RC, Dang R, Sargent JD. Association Between Initial Use of e-Cigarettes and Subsequent Cigarette Smoking Among Adolescents and Young Adults: A Systematic Review and Meta-analysis. JAMA Pediatr. 2017 Aug 1;171(8):788-797. doi: 10.1001/jamapediatrics.2017.
Chaffee BW, Watkins SL, Glantz SA. Electronic Cigarette Use and Progression From Experimentation to Established Smoking. Pediatrics. 2018 Apr;141(4). pii: e20173594. doi: 10.1542/peds.2017-3594. Epub 2018 Mar 5.
Watkins SL, Glantz SA, Chaffee BW.Association of Noncigarette Tobacco Product Use With Future Cigarette Smoking Among Youth in the Population Assessment of Tobacco and Health (PATH) Study, 2013-2015. JAMA Pediatr. 2018 Feb 1;172(2):181-187. doi: 10.1001/jamapediatrics.2017.4173.
Soneji SS, Sung HY, Primack BA, Pierce JP, Sargent JD. Quantifying population-level health benefits and harms of e-cigarette use in the United States. PLoS One. 2018 Mar 14;13(3):e0193328. doi: 0.1371/journal.pone.0193328. eCollection 2018.
National Academies of Sciences, Engineering, and Medicine. 2018. Public health consequences of e-cigarettes. Washington, DC: The National Academies Press. Doi:https://doi.org/10.17226/24952. Available at: http://nationalacademies.org/hmd/Reports/2018/public-health-consequences-of-e-cigarettes.aspx

Add new comment