February 24, 2014
Stanton A. Glantz, PhD
Public comment on FDA's process of documenting substantial equivalence: Need high standards of evidence and real transparency
We just submitted this comment to the FDA:
COMMENT SUBMITTED IN RESPONSE TO FDA ON PROPOSED COLLECTION OF INFORMATION REGARDING SECTION 905(j) REPORTS: DEMONSTRATING SUBSTANTIAL EQUIVALENCE FOR TOBACCO PRODUCTS
Docket No. FDA-2013-N-1558
1jy-8an3-hgzl
Stanton Glantz, PhD
Lauren Lempert, JD, MPH
Center for Tobacco Control Research and Education
University of California San Francisco
February 25, 2014
We submit these comments in response to FDA’s notice concerning the proposed collection of information regarding Section 905(j) Reports: Demonstrating Substantial Equivalence for Tobacco Products. We previously submitted public comments on substantial equivalence in reference to dockets 2010-D-0635 and 2013-N-1588, which are incorporated by reference in this comment.[1]
1. The proposed collection of information is necessary for the proper performance of FDA’s functions.
The fundamental purpose of the Food and Drug Administration’s (FDA) regulatory authority over tobacco products granted by the Family Smoking Prevention and Tobacco Control Act (FSPTCA)[2] is to protect the public health. The purposes of this law include providing FDA regulatory authority with respect to the manufacture, marketing, and distribution of tobacco products,[3] the authority to address issues of particular concern to public health officials,[4] and the authority “to ensure that consumers are better informed, to require tobacco product manufacturers to disclose research which has not previously been made available, as well as research generated in the future, relating to the health and dependency effects or safety of tobacco products.”[5] In order to properly perform these functions, it is therefore essential that FDA collect adequate information in order to determine whether tobacco products are appropriate for the public health, and to disclose that information to the public so that consumers and public health officials are better informed as required by FSPTCA.
The substantial equivalence pathway laid out in section 905(j) of the FSPTCA that allows a tobacco product manufacturer to sidestep the premarket application process required by section 910 before introducing a new tobacco product into interstate commerce created a potential loophole that allows manufacturers to continue to market products placed on the market before March 22, 2011 and for which substantial equivalence reports were submitted prior to March 23, 2011 unless and until FDA issues an order that the tobacco product is not substantially equivalent to a predicate product.[6] On February 21, 2014, FDA announced that it had taken its first action requiring four tobacco products to be removed from the market on the grounds that they did not demonstrate substantial equivalence to predicate products.[7] While this is an important first step, 3,513 tobacco products currently remain on the market awaiting FDA’s determinations on their provisional substantial equivalence applications.[8] This means that until FDA determines that these products are NOT substantially equivalent and orders their removal from the market, there are more than 3,500 products on the market that have the potential to increase the likelihood that nonusers will begin using these products or that will decrease the likelihood that current users will quit using these products, contributing to the 480,000 deaths caused by tobacco products each year and to the more than 3,200 people younger than 18 years old who each day try their first cigarette.[9]
The proposed collection of information is essential for FDA to fulfill its mandated function to adequately review these applications and protect the public from the continued marketing of existing tobacco products and the introduction of new tobacco products that are attracting new and young smokers and maintaining or aggravating current smokers’ addictions to these lethal products.
2. Tobacco manufacturers must meet the burden of demonstrating substantial equivalence clearly and precisely.
Section 905(j) and the Guidance for Section 905(j) Reports: Demonstrating Substantial Equivalence for Tobacco Products (Guidance) make clear that the burden is on the tobacco manufacturer to submit a 905(j) report that provides FDA with “sufficient information to enable FDA to determine whether the new tobacco product is (1) substantially equivalent, within the meaning of section 910(a)(3) of the Act, to an appropriate predicate product, and (2) in compliance with the requirements of the Act (section 910(a)(2)(A) of the Act).”[10] FDA must hold tobacco manufacturers accountable to this requirement, and must not let these companies shift the burden to FDA to in effect prove that they are not substantially equivalent.
However, proving substantial equivalence is not sufficient; even if a manufacturer meets the first prong of the burden and demonstrates that their new product is substantially equivalent to a predicate product, they must still meet the second part of the burden and show that the new product is otherwise in compliance with the requirements of the FSPTCA.
To meet this burden, all data and information submitted to FDA must be clear and specific, and the side-by-side quantitative comparisons of the new tobacco products with the predicate product must include exact measurements and descriptions of design features, ingredients, additives, harmful and potentially harmful constituents, including smoke constituents, and materials, as well as explicit descriptions of heating sources and composition. FDA must require that all reports and data submissions have adequate statistical power and effect size to ensure that FDA is not evaluating misleading data.[11]
The Guidance states that FDA “may” request additional data including Consumer Perception Studies that provide “data comparing consumer perceptions with respect to the new tobacco product and the predicate that could affect initiation, cessation, frequency of use, patterns of use, smoking behavior, and perceptions of harm or addictiveness.”[12] It is axiomatic that consumer perceptions are the key influence on their purchases, and therefore are a primary focus of the tobacco industry’s marketing and packaging efforts. Therefore, Consumer Perception Studies, including the tobacco manufacturer’s market research studies, should be required, not merely occasionally requested, for every substantial equivalence application, and should be held to the same exacting standards as the side-by-side qualitative and quantitative data required for other ingredients, additives, materials, and product design features.
Similarly, an adequate summary section containing a description of the specific similarities and differences between the new tobacco product and the predicate product should be required, rather than “strongly recommended”, to be included in the 905(j) report and should be made available to the public.
Consumers and public health officials should not be given the burden of chasing after tobacco product manufacturers to provide this information, and then, as we were, forced to make a Freedom of Information Act request to the FDA to get this information.[13] If the FDA were truly interested in reducing the burden of collection of information, they would minimize the necessity of having to additionally respond to FOIA requests by requiring summary information to be submitted by manufacturers at the outset.
3. FDA must be transparent.
One of the four stated priorities of the Center for Tobacco Products is to be transparent and “provide the public with accurate information on the contents of tobacco and the consequences of tobacco use.”[14] Since 905(j) reports are submitted digitally, they should be made easily available to the public. However, these reports cannot and do not provide the public with adequate or accurate information if they are so heavily redacted that the public cannot access meaningful information. FDA should not routinely accept tobacco manufacturers’ broad and indiscriminate claims that the information they submit to FDA are trade secrets and therefore must not be disclosed to the public; rather, FDA should make manufacturers substantiate their trade secret claims.
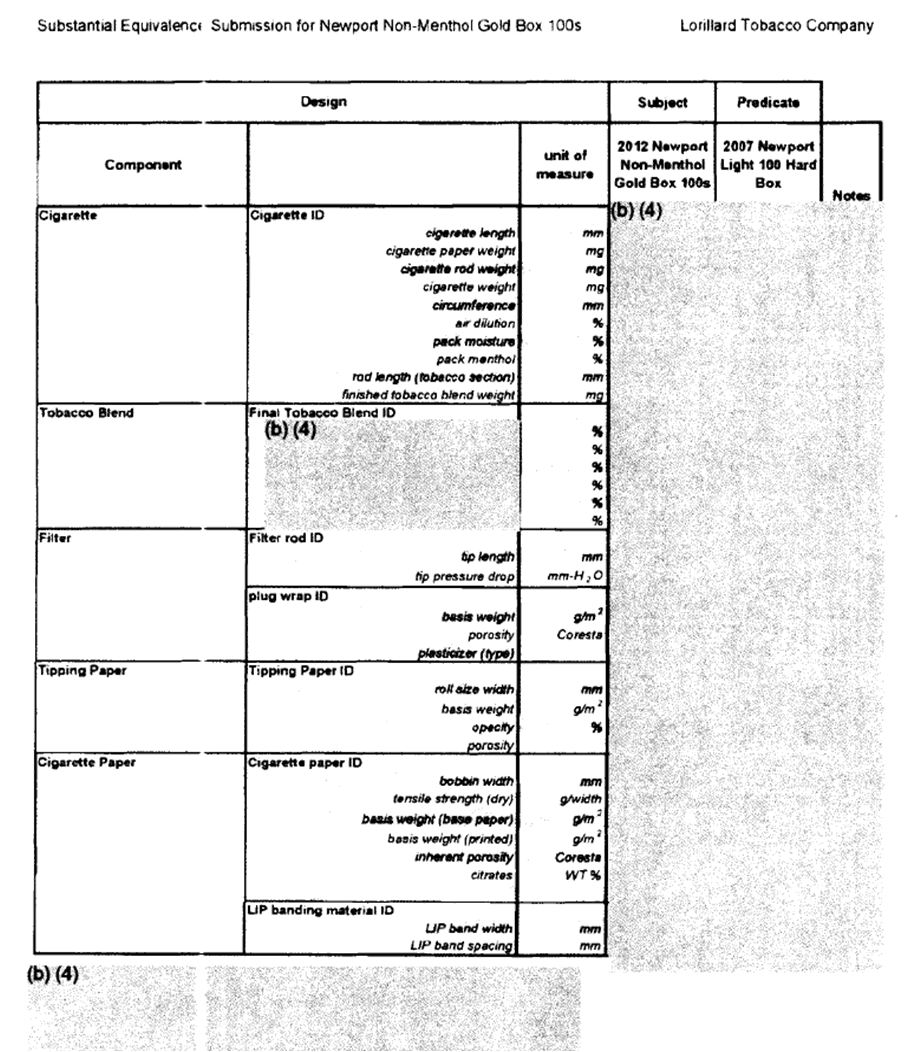
For example, in response to our FOIA request for Lorillard substantial equivalence application for Newport Non-Menthol Gold cigarettes, FDA provided 197 pages of heavily redacted information that made it virtually impossible to understand how the new product compared with the predicate product.[15] While some of the redacted information would qualify as trade secrets, there is no legal justification for redacting information such as the length of the cigarette, which does not legally qualify as a trade secret as this information is readily ascertainable by any consumer or competitor who buys the product on the open market.[16] Since tobacco companies routinely engage in extensive reverse engineering of their competitors’ products, most of the other information that has been redacted would not qualify as trade secrets either, as the information is neither secret nor commercially valuable once broadly disseminated throughout the industry. The information is only being kept secret from the public, in violation of FDA’s stated goals of transparency and protecting the public health.
FDA should prioritize review of the 3,513 provisional applications to ensure that tobacco products already on the market are truly “substantially equivalent” to predicate products. The proposed collection of information will allow FDA to do this and achieve its regulatory mandate to protect the public health and its goal of transparency.
Figure: Page from the FDA’s response to our FOIA request on how they determined that Lorillard’s SE application warranted approval. The “(b)(4)” redactions refer to the trade secret exemption in FOIA.
[1] Glantz S. RE: Guidance for Industry and Food and Drug Administration Staff; Section 905(j) Reports: Demonstrating Substantial Equivalence for Tobacco Products; Availability (Document ID FDA-2010-D-0635-0001). December 15, 2012. Available on www.regulation.gov at 1jw-82jq-ae81; Glantz S. Comment Regarding Power and Effect Size in Guidance for Industry and Food and Drug Administration Staff; Section 905(j) Reports: Demonstrating Substantial Equivalence for Tobacco Products; Guidance FDA-201-D-0635, January 9, 2013; and Glantz S and Lempert L. Comment Submitted in Response to FDA on Proposed Collection of Information Regarding Exemptions from Substantial Equivalence requirements for Tobacco Products, February 17, 2014.
[2] Pub.L. 111-31, H.R. 1256 (June 22, 2009)
[3] Family Smoking Prevention and Tobacco Control Act section 3(1)
[4] Family Smoking Prevention and Tobacco Control Act section 3(2)
[5] Family Smoking Prevention and Tobacco Control Act section 3(6)
[6] Family Smoking Prevention and Tobacco Control Act sections 905(j), 910(a)(2)(B); Guidance for Industry and FDA Staff- Section 905(j) Reports: Demonstrating Substantial Equivalence for Tobacco Products, January 5, 2011.
[7] FDA news release: FDA issues first orders to stop sale, distribution of tobacco products. February 21, 2014. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm386707.htm?...
[8] See FDA website: Total number of product submissions received or filed in the month. http://www.accessdata.fda.gov/FDATrack/track?program=ctp&id=CTP-OS-total...
[9] U.S. Dept. of Health and Human Services. The Health Consequences of Smoking – 50 Years of Progress: A Report of the Surgeon General. (2014); U.S. Department of Health and Human Services.Preventing Tobacco Use Among Youth and Young Adults: A Report of the Surgeon General.(2012); Centers for Disease Control and Prevention. Youth and Tobacco Use. http://www.cdc.gov/tobacco/data_statistics/fact_sheets/youth_data/tobacco_use/
[10] Guidance for Industry and FDA Staff- Section 905(j) Reports: Demonstrating Substantial Equivalence for Tobacco Products, January 5, 2011, p. 7.
[11] See our previous comment submitted on this point: Glantz S. Comment Regarding Power and Effect Size in Guidance for Industry and Food and Drug Administration Staff.
[12] Guidance for Industry and FDA Staff- Section 905(j) Reports: Demonstrating Substantial Equivalence for Tobacco Products, January 5, 2011, p. 12.
[13] Glantz S. Letters to Neil Wilcox, Lorillard re: Submissions Tracking Numbers (STN) SE003730 and SE003731, July 8, 2013, https://www.tobacco.ucsf.edu/sites/g/files/tkssra4661/f/u9/SAG%20letters%20to%20Lorillard.pdf; Wilcox N. Letter to Stanton Glantz, UCSF re: Submissions Tracking Numbers (STN) SE003730 and SE003731, July 17, 2013, https://www.tobacco.ucsf.edu/sites/g/files/tkssra4661/f/u9/Lorillard%20-%20response%20letter%20071713.pdf; Glantz S. Letters to Freedom of Information Act Officer, FDA re Submissions Tracking Numbers (STN) SE003730 and SE003731, August 2, 2013, https://www.tobacco.ucsf.edu/sites/g/files/tkssra4661/f/u9/FDA-Lorilla....
[14] FDA website: About FDA/Transparency/Basics: What are the priorities of the Center for Tobacco Products? http://www.fda.gov/AboutFDA/Transparency/Basics/ucm194435.htm
[15] FDA FOIA officer, Scanned Substantial Equivalence applications, correspondence, and combined reviews, August 13, 2013.
[16]Page from FDA FOIA officer, Scanned Substantial Equivalence applications, correspondence, and combined reviews, August 13, 2013. See Figure.

Add new comment